Learning Outcomes:
i. Students will grasp the experimental techniques for measuring heat of fusion and heat of vaporization.
ii. Comprehend the concept of latent heat and recognize its significance in phase changes.
iii. Analyze temperature-time graphs obtained during heating ice and boiling water to calculate heat of fusion and heat of vaporization.
iv. Apply the knowledge of heat of fusion and heat of vaporization to explain the energy requirements for various processes, such as melting ice or boiling water.
v. Appreciate the importance of accurate measurements and data analysis in determining heat of fusion and heat of vaporization.
Introduction:
As we observe the transformation of ice into liquid water or the steam rising from a boiling pot, we witness intriguing examples of phase changes, where substances transition from one state of matter to another. These transformations involve energy transfers, and the concepts of heat of fusion and heat of vaporization provide a quantitative measure of the energy required for these changes to occur. This lesson delves into the experimental methods for measuring heat of fusion and heat of vaporization, exploring the significance of these values and their practical applications.
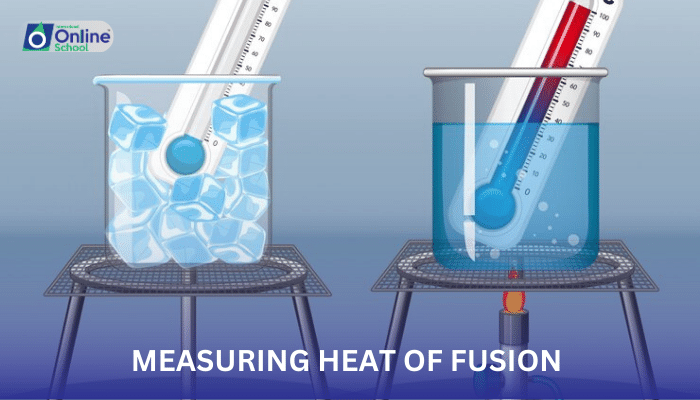
i. Measuring Heat of Fusion: Unveiling the Energy of Melting
Experimental Setup: A calorimeter, a device designed to measure heat transfer, is filled with a known mass of ice. A thermometer is placed in the ice to monitor temperature changes.
Heating Process: Heat is supplied to the calorimeter by placing it in a warm water bath or using an electrical heater.
Temperature-Time Graph: The temperature of the ice-water mixture is recorded at regular intervals and plotted against time.
Heat of Fusion Calculation: The heat of fusion is calculated by considering the horizontal portion of the temperature-time graph, where the temperature remains constant during the melting process. The heat absorbed during this phase change represents the energy required to overcome the intermolecular forces and melt the ice.
ii. Measuring Heat of Vaporization: Quantifying the Energy of Boiling
Experimental Setup: A calorimeter, containing a known mass of water, is placed on a heat source, such as a hot plate or a Bunsen burner. A thermometer is placed in the water to monitor temperature changes.
Boiling Process: Heat is supplied to the calorimeter, causing the water to boil.
Temperature-Time Graph: The temperature of the water is recorded at regular intervals and plotted against time.
Heat of Vaporization Calculation: The heat of vaporization is calculated by considering the horizontal portion of the temperature-time graph, where the temperature remains constant during the boiling process. The heat absorbed during this phase change represents the energy required to overcome the intermolecular forces and vaporize the water.
iii. Interpreting Temperature-Time Graphs: Unveiling Latent Heat
The horizontal portions of the temperature-time graphs represent regions of constant temperature during phase changes. These regions indicate that the energy supplied is being used to break the intermolecular forces and change the state of matter, rather than increasing the temperature of the substance. The energy absorbed during these phases is referred to as latent heat, representing the energy required to bring about the specific phase change.
iv. Real-World Applications: Heat of Fusion and Vaporization at Work
Heat of fusion and heat of vaporization have numerous applications in various fields:
Refrigeration and Air Conditioning: Understanding heat of vaporization is crucial in designing and operating refrigeration systems and air conditioners, which utilize the principle of latent heat absorption to cool environments.
Energy Efficiency and Conservation: Heat of fusion and heat of vaporization play a role in energy efficiency and conservation efforts, as they represent the energy required for various industrial processes and household appliances.
Material Science: The heat of fusion and heat of vaporization of materials are important properties in determining their thermal behavior and suitability for various applications.
Heat of fusion and heat of vaporization, fundamental concepts in physics, provide insights into the energy transformations that occur during phase changes. By understanding the experimental methods for measuring these values, analyzing temperature-time graphs, and appreciating the significance of latent heat, we gain a deeper understanding of the energy requirements for various processes and the applications of these concepts in diverse fields. As we explore the intricate world of phase changes, we marvel at the precision of experimental measurements and the profound impact of heat of fusion and heat of vaporization on our physical world.